Montana GranuFlo Recall Lawsuits Filed for Heart Attacks - Attorneys Handling Montana GranuFlo Lawsuits Offer No-Cost, No-Obligation GranuFlo Case Review
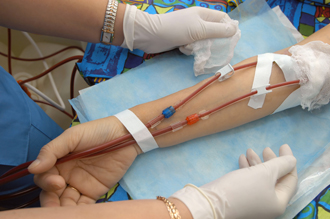
Naturalyte GranuFlo Acid Concentrate and Liquid Acid Concentrate are the subjects of Montana GranuFlo recall lawsuits based on reports that the products greatly increase a patient’s risk for heart attack. Products of the German company Fresenius Medical Care, Naturalyte GranuFlo and Liquid Acid Concentrates fall into the class of dialysates and are used in dialysis treatment to cleanse the blood in conjunction with a dialysis machine. Fresenius Medical Care is the subject of an FDA investigation due to the company’s failure to warn the public of the GranuFlo heart attack risk when it was discovered in 2011. GranuFlo, which was designed to neutralize blood acidity, has a tendency to increase bicarbonate levels to a hazardous degree, making patients six times more likely to experience heart attack. GranuFlo was used in both Fresenius dialysis clinics and other clinics. Fresenius Medical Care is the nation's leading provider of dialysis clinics, machines and other dialysis products.
This website offers comprehensive information about GranuFlo heart attack risks and Montana GranuFlo lawsuits, including why GranuFlo is used in dialysis, what causes GranuFlo to be dangerous, where to find information about the GranuFlo FDA recall, what you might expect from a GranuFlo heart attack lawsuit, and how to contact a Montana GranuFlo attorney.

GranuFlo Heart Attack Risk - Information for Montana Residents
GranuFlo FDA Recall - Information for Montana Residents

The FDA issued a GranuFlo Heart Attack Warning on May 25, 2012, which was followed by a Class 1 FDA GranuFlo Recall on June 27, 2012. Based on data collected at Fresenius Medical Care dialysis clinics in 2010, the FDA GranuFlo Recall warns that GranuFlo “may cause serious adverse health consequences, including death.” Class 1 recalls are the FDA's most severe level of medical recall and are used to warn medical professionals and the public of serious dangers associated with drugs. The FDA is now conducting further research to determine the full extent of GranuFlo’s dangers. Read here to learn what the GranuFlo FDA warning says, why the FDA is conducting more research and who is affected by the risk of GranuFlo heart attacks.

Montana GranuFlo Recall Lawsuits
Montana GranuFlo lawsuits may be filed by or on behalf of persons who have had a heart attack immediately after GranuFlo was used in their dialysis treatment. Fresenius Medical Care is under FDA investigation for neglecting to disclose information regarding the risk of GranuFlo heart attack, specifically that the company found that elevated levels of bicarbonate from taking GranuFlo increased a patient's risk of heart attack six-fold. Failure to disclose this information publicly may constitute a violation of federal drug safety regulations. Our Montana GranuFlo lawsuit information page will answer many of your questions as well as provide a no-cost, no-obligation Montana GranuFlo lawsuit case review.


 OnderLaw, LLC -
OnderLaw, LLC -